Anti-inflammatory activity of chondroitin sulfate
Summary
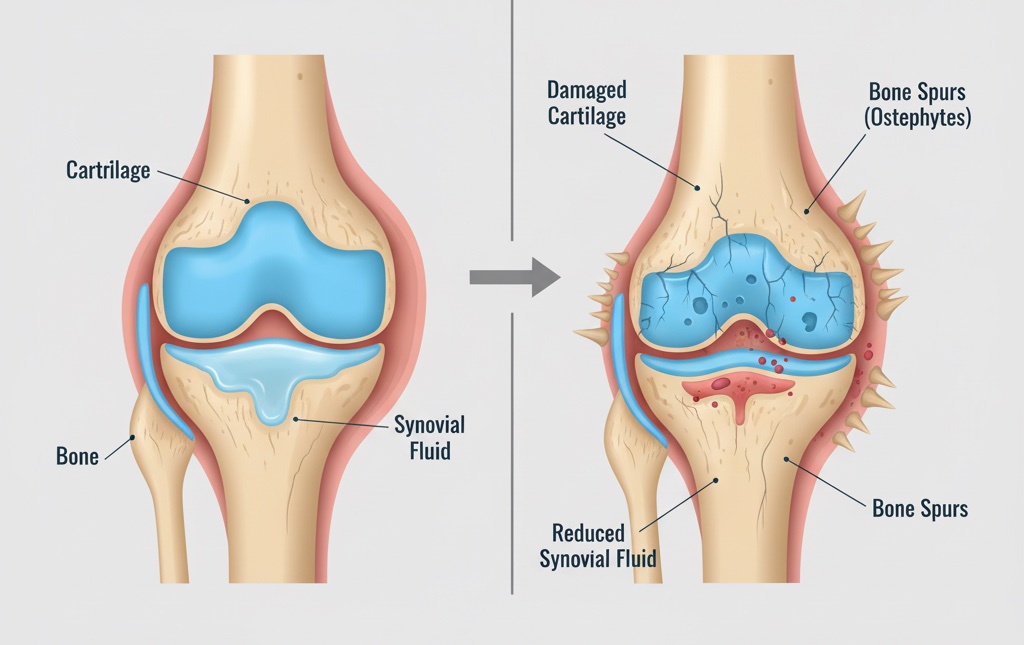
Osteoarthritis is primarily characterized by areas of destruction of articular cartilage and by synovitis. Articular damage and synovitis are secondary to local increase of pro-inflammatory cytokines (interleukin-1β and tumor necrosis factor-α), enzymes with proteolytic activity (matrix metalloproteinases), and enzymes with pro-inflammatory activity (cyclooxygenase-2 and nitric oxide synthase-2). Enhanced expression of these proteins in chondrocytes and in synovial membrane appears associated to the activation and nuclear translocation of nuclear factor-κB (NF-κB). Chondroitin sulfate (CS) prevents joint space narrowing and reduces joint swelling and effusion. To produce these effects, CS elicits an anti-inflammatory effect at the chondral and synovial levels. CS and its disaccharides reduce NF-κB nuclear translocation, probably by diminishing extracellular signal-regulated kinase1/2, p38mitogen-activated protein kinase and c-Jun N-terminal kinase activation. This review discusses the evidence supporting that CS pleiotropic effects in chondrocytes and synoviocytes are primarily due to a common mechanism, e.g., the inhibition of NF-κB nuclear translocation.
Key words
Chondroitin sulfateDisaccharidesOsteoarthritisInflammationNF-kBSignal transduction
Osteoarthritis
Osteoarthritis is characterized by focal areas of loss of articular cartilage, with varying degrees of osteophyte formation, subchondral bone change and synovitis1. The pathophysiology of osteoarthrosis remains controversial. It has been proposed that continuous use of the joint implies multiple microtrauma to the articular cartilage and formation of fibronectin and extracellular matrix fragments (EMFs). Fibronectin fragments (FN-f) contribute to cartilage destruction2 by binding to α5β1 integrin receptor of the chondrocyte with the subsequent activation of protein kinase C (PKC), proline-rich tyrosine kinase-2, extracellular signal-regulated kinase1/2 (ERK1/2), p38mitogen-activated protein kinase (p38MAPK) and c-Jun N-terminal kinase (JNK), that trigger the nuclear translocation of activated protein-1 (AP-1) and nuclear factor-κB (NF-κB), and enhanced expression of matrix metalloproteinases (MMPs), MMP-3 and MMP-133. MMPs will cleave the EMFs.
In chondrocytes, increased expression of MMPs is accompanied by an enhanced synthesis of pro-inflammatory cytokines, essentially interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), which will sustain the activation of chondrocytes and moreover, will further promote the formation of MMPs, aggrecanase, reactive oxygen intermediates, nitric oxide, and lipid-derivative inflammatory mediators such as prostaglandins and leukotrienes; these substances will enhance the catabolic activity of the chondrocytes and cause the destruction of the cartilage matrix. On the other hand, EMFs, IL-1β and TNF-α released into the synovial fluid will activate macrophages, mastocytes and synoviocytes in the synovial membrane originating the synovitis. Activation of synovial cells will result in further release of IL-1β, TNF-α and MMPs that will contribute to the destruction of the cartilage matrix.
There is clinical evidence showing that osteoarthritis and synovitis are associated. Synovial abnormalities are detectable in 50% of patients with osteoarthrosis. Synovitis is reflected by several of the signs and symptoms of osteoarthritis, such as swelling and effusion, redness, pain and stiffness7. Moderate or large effusions and synovial thickening are more frequent among patients with knee pain than those without pain, suggesting that these signs are associated with the pain of osteoarthritic knee; furthermore, the severity of knee pain is associated with synovial thickening8. In patients with osteoarthritis, changes in pain are closely associated to the changes in synovitis but not to cartilage loss9. The presence of synovitis at early stages of osteoarthritis is associated with a more rapid and destructive progression of the disease7. Finally, there is evidence that a subset of patients with osteoarthritic joint disease present synovitis and synovial hyperplasia without cartilage damage and EMFs, suggesting that in some patients, synovitis is a very early or the initial event in the development of osteoarthritis10. Independently of the sequence of events in the apparition of osteoarthritis, e.g., cartilage damage or synovitis at the origin of osteoarthritis, cartilage damage and synovitis are present in a great proportion of patients, both contribute to the signs and symptoms of osteoarthritis, and both should be the target of therapy.
Chondroitin sulfate (CS) in osteoarthritis, clinical evidence
Randomized clinical trials have shown that CS reduces pain and improves articular function11, 12, 13, reduces joint swelling and effusion14, and prevents joint space narrowing of the knee11, 13 and fingers15, 16 more effectively than placebo. According to these effects, CS has been classified as a symptomatic slow acting drug in osteoarthritis (SYSADOA) and a structure/disease modifying anti-osteoarthritis drug (S/DMOAD)11, 15.
Effect of CS on articular cartilage, mechanism of action
The complex clinical response to CS may tentatively be explained by the numerous effects elicited by CS. On the one hand, the decrease in pain and swelling may be explained by an anti-inflammatory effect of CS, probably through diverse mechanisms such as diminishing the expression of phospholipase A2 (PLA2)17, of cyclooxygenase-2 (COX-2), and the concentrations of prostaglandin E2 (PGE2)18, 19. Moreover, in joints CS reduces the concentrations of pro-inflammatory cytokines, such as TNF-α20 and IL-1β21, and systemic and joint concentrations of NO19, 22 and of reactive oxygen species (ROS)20. On the other hand, protection of the joint structure may be explained by the fact that in chondrocytes, CS diminishes IL-1β-mediated increase in MMP-218, MMP-318, MMP-918, 19, 21, MMP-1318, 19, and MMP-1418. Moreover, it has been documented that hyaluronan and mixtures of low concentrations of CS and glucosamine are able to prevent the release of MMP-3 and MMP-13 triggered by FN-f23, 24. Finally, in subchondral bone, CS increases osteoprotegerin (OPG) and reduces the expression of receptor activator of NF-κB ligand (RANKL), effects that may result in the reduction of the resorptive activity in subchondral bone25.
In chondrocytes, CS diminishes ERK1/2 phosphorylation and abrogates the phosphorylation of p38MAPK induced by IL-1β; as a consequence, CS reduces IL-1β-induced NF-κB nuclear translocation. However, CS does not reduce IL-1β-induced AP-1 nuclear translocation. On the other hand, CS decreases nitroprusside-induced apoptosis of the chondrocytes probably by preventing p38MAPK activation26. In chondrocytes, chondroitin disaccharides sulfated at positions 4 and/or 6, (1-4)-O-(d-glucopyranosyluronic acid)–(1-3)-O-(2-N-acetamido-2-deoxy-d-galactopyranosyl-4/6-sulfate) (Δdi-4S, Δdi-6S and Δdi-4,6S) reduce IL-1β-induced NF-κB nuclear translocation to a similar extent as CS, e.g., Δdi-4S, Δdi-6S and Δdi-4,6S reduce NF-κB translocation by 11, 13 and 17%, respectively (P < 0.05, N = 9)
It has been widely documented in the chondrocytes that IL-1β-induced increase in expression of MMP-327, MMP-928, MMP-1327, 29, 30, COX-230, 31, nitric oxide synthase-2 (NOS-2), IL-1β and TNF-α32 is mediated by the activation and nuclear translocation of NF-κB and AP-1. Moreover, there is evidence that the activation of PLA2 requires the activation of p38MAPK and ERK1/231, and that the induction of RANKL expression requires the activation of ERK1/2 and phosphatidylinositol 3-kinase/protein kinase B (PI-3K/Akt) pathways33. Since the above mentioned effects of IL-1β in the chondrocyte are mediated by the activation of p38MAPK and of ERK1/2, and the nuclear translocation of NF-κB, it is tempting to speculate that the pleiotropic effects of CS are dependent, at least in part, by its ability to inhibit p38MAPK and ERK1/2 phosphorylation and NF-κB nuclear translocation.
Effect of CS on the synovial membrane, mechanism of action
Synovial tissue from patients with early osteoarthritis shows activated fibroblast-like synoviocytes (FLS), macrophages, T lymphocytes, and mast cells infiltration34. FLS release IL-1β, IL-6, IL-8, MMP-1, MMP-2, MMP-3, MMP-13, MMP-14, MMP-16, tissue inhibitor of metalloproteinases-1 (TIMP-1), RANKL, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF)4.
There is evidence supporting that the role of NF-κB in the development of synovitis appears pivotal. In FLS, the production of IL-1β, IL-6, IL-8, and MMP-1, MMP-3, requires the activation and nuclear translocation of NF-κB35, 36. Moreover, the activation of NF-κB increases FLS proliferation and changes the phenotype of these cells to highly invasive FLS with great motility and ability to secrete cytokines and MMP-1337. Inhibition of the IκB kinase (IKK) complex impedes the phosphorylation of the inhibitor of κB (IκBα) and as a consequence, prevents NF-κB activation. In synovial macrophages, inhibition of IKK diminishes IL-1β-induced production of IL-6; moreover, in rats with adjuvant-induced arthritis, intra-articular injection of a specific IKK-β inhibitor reduces arthritis activity and bone destruction; synovial inflammation was also decreased as documented by the reduction in synovial cellularity, TNF-α, IL-1-β concentrations, and reduction of the volume of the paw38. The role of NF-κB in the initiation of synovitis was further supported by administering in the articulation of the rat with adjuvant-induced arthritis a dominant-negative form of IKK-β that reduced synovial cellularity by 50%, and diminished synovial concentrations of IL-1β, TNF-α and MMP-339. These results provide evidence that activation and nuclear translocation of NF-κB is an important step in the development of synovitis.
There is little information about the effect of osteoarthritis treatment with CS on synovitis manifestations, e.g., joint swelling and effusion. The multicenter, double-blind, placebo- and celecoxib-controlled Glucosamine/chondroitin Arthritis Intervention Trial (GAIT) assessed the effect of CS and glucosamine alone or in combination on joint swelling and/or effusion in 1583 patients with mild to severe knee osteoarthritis14. The patients received 1200 mg of CS, or 1500 mg of glucosamine or both CS and glucosamine, or 200 mg of celecoxib or placebo, daily for 24 weeks. The trial demonstrates that CS diminished the percentage of patients with signs of synovitis (joint swelling and effusion) from 28.3% at baseline to 12.4% at the end of 24 weeks of treatment (P = 0.01, N = 307). It is of interest that the beneficial effect of CS (P = 0.02, N = 248) was observed in the patients with mild pain (Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores 125–300). In patients with moderate to severe pain (WOMAC pain scores 301–400) receiving CS, the percentage of patients with swelling and/or effusion tended to decrease from 30.0% at baseline to 14.9% (P = 0.3, N = 67) at the end of follow-up.
Further supporting that CS reduces the signs and symptoms of synovitis, a study showed that intra-articular injection of hyaluronate, a glycosaminoglycan with a molecular weight of 8.4 × 105, to patients with rheumatoid arthritis improves local clinical symptoms, decreases synovial fluid, reduces PGE2 concentrations and diminishes pain40.
Several animal studies demonstrate that CS reduces the signs and symptoms of synovitis. In DBA/1J mice with a type II collagen-induced arthritis, treated for 9 weeks with various dosages of CS, the infiltration of inflammatory cells, granulated tissue formation, proliferation of synovial lining cells, paw edema and destruction of articular cartilage were partially prevented by treatment with 1000 mg/kg/day of CS for 63 days41. In dogs with unilateral carpal synovitis induced by injecting the right radiocarpal joint with chymopapain, prior treatment with CS reduces the extend of synovitis42. In rabbits with experimental osteoarthritis, intra-articular administration of N-acetylglucosamine elicited an anti-inflammatory effect and suppressed the synovitis43.
All these studies strongly support that in animal models and in humans, glycosaminoglycans reduce the signs and symptoms of synovitis. The mechanism of action underlying the reduction of synovitis by CS and other glycosaminoglycans remains incompletely characterized. It has been reported that CS disaccharide Δdi-6S reduces IL-1β-induced nuclear translocation of NF-κB by 67% in synoviocytes44. This observation is in agreement with the effect of CS and its Δdi-4S and Δdi-6S disaccharides in chondrocytes, e.g., they reduce NF-κB nuclear translocation. Since oral CS increases plasma concentrations of Δdi-4S and Δdi-6S45, it is conceivable that in humans, the decrease in synovitis signs produced by CS may be explained, at least in part, by the reduction in NF-κB nuclear translocation in synoviocytes and macrophages, with the subsequent diminution of activation of these cells and decrease in synovitis.
In summary, CS and/or the sulfated disaccharides appear to elicit an anti-inflammatory effect at the synovial membrane and chondrocytes levels. Possibly, CS and/or disaccharides reduce the inflammatory reaction by diminishing NF-κB nuclear translocation (Fig. 4). In the chondrocytes, this effect is mediated by the inhibition of p38MAPK phosphorylation and to a minor degree ERK1/2 phosphorylation. Indeed, further studies are required to better characterize the precise mechanism of action underlying CS-induced improvement of synovitis.
2025 Summit Nutritionals